The FHSC Global Registry now has approximately 62.5K cases registered from 62 contributing countries thus far and continues to receive new cases and is expanding to other countries. The FHSC currently consists of 90 Lead Investigators spanning 71 countries.
The EAS FH Studies Collaboration (FHSC) was established to empower the medical and world-wide FH community to achieve global policy change in how FH is detected and managed.
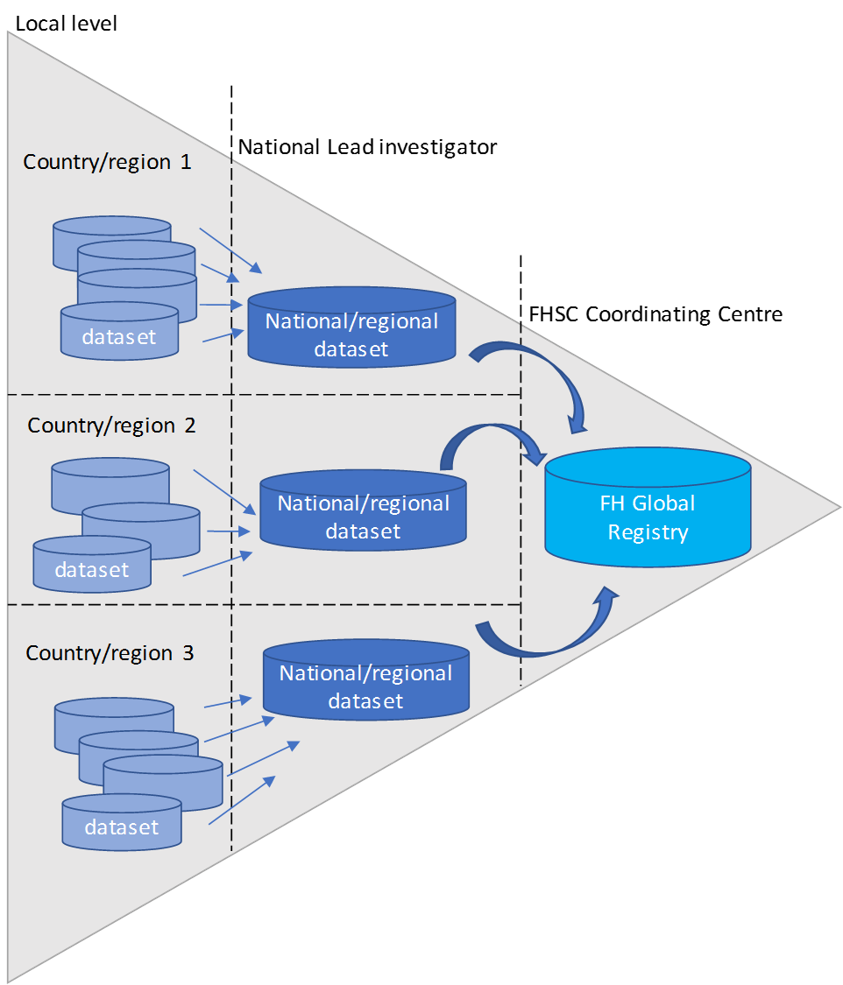
FHSC has established the first global FH Registry, an international secure warehouse of FH Data, collected and merged from all the countries (currently ca. 70) included in the network. This registry is the first example of Big Data on FH currently available.
The Registry is coordinated by Prof. K. Ray and his team, and hosted securely at Imperial College London (UK), in strict adherence with all data safety protocols and regulatory requirements (GDPR).
FHSC relies on a centralised data management system that has been developed and tested over the past years, in order to design and customise an IT platform that matches the requirements of security and facility in handling a huge amount of incoming data.
The participants in the FH Studies Collaboration meet every year to share information and experiences in an annual Steering Committee meeting, held in conjunction with the EAS Congress.
Setting up the registry
The first step was to establish a consortium of major FH registries across Europe, Asia-Pacific, Africa and South America with access to readily available data on individuals with FH. Key opinion leaders in the field agreed to contribute to this database and other international collaborators were approached for collaboration and contribution.
The initial step involved collecting for each region details such as:
- the number of FH databases in their own country/region, and the approximate number of FH patients included in these registries;
- how these patients (and their relatives) are being managed;
- who pays for their treatment (i.e. whether government, private insurer, or self-funded);
- what is their local/regional/national policy on cascade screening for relatives, and the availability and utilization rates of genetic testing; and
- who else in their opinion plays an important role in FH management in their region/country (who may then be contacted for potential collaboration).
Merging and harmonisation of data
A detailed data request form was sent to consenting investigators for them to provide pseudonymised data on individuals with FH. After ensuring satisfactory data quality from all registries, the information was merged and harmonised into the central database of the FHSC using bespoke algorithms. This process allows to overcome the diversity and heterogeneity of data coming from multiple cohorts in the world.
FHSC IDEAPP
FHSC Coordinating Centre have developed a web-based platform called FHSC Individual Data Entry Application (IDEAPP) exclusive to FHSC National Lead Investigators and their collaborators cost-free to support local data entry, management and sharing with the FHSC Global Registry.
The advanced features of FHSC IDEAPP enables variables entered to automatically process and harmonise within the platform to provide a uniform format.
FHSC IDEAPP has been well received by its users and in several cases has enabled FHSC National Lead Investigators to start their own local/national registry from afresh at a zero cost.
Now the registry is established
As per December 2021, the FH Registry includes more than 62,500 cases from 62 countries from all parts of the world. New countries and investigators are constantly joining, contributing to the Registry with their data and expanding the reach of the network. Exploratory analyses are conducted on the harmonised dataset to study various cross-sectional relationships. Particular emphasis has been placed on exploring differences between:
- HoFH and HeFH;
- different genetic subtypes of FH (e.g. broadly whether due to mutations in the LDL receptor, ApoB, PCSK9 or LDLRAP pathways);
- FH treated by a generalist vs. specialist;
- FH with pre-existing CVD vs. those without;
- FH with a family history of premature CVD vs. those without, and
- FH individuals who attain LDL-C targets on standard treatments vs. those who do not.
- For analysis of risk (of incident CVD outcomes or death), information collected to date is analysed as if the study were a retrospective cohort study
What we expect to learn
Sharing and pooling the data will help reduce redundant research, avoid duplication of efforts and reduce costs of research; it will allow to assess questions which cannot be addressed in individual studies, provide greater precision than currently possible, facilitate exploratory approaches due to the large sample size and to suggest new hypothesis.
Amongst others, we expect to learn:
- Usual demographic characteristics (including numbers of siblings/ children both affected and unaffected if known), genotype (if available), treatments, baseline lipids and on- treatment lipids will be obtained from individual studies.
- Where individuals have data on HoFH with apheresis, information will be sought on frequency and the mean interval LDL-C levels. CVD events (non-fatal MI or stroke) will be obtained from health records or consultations, and case fatalities from national registers.
- Continuous variables will be compared with parametric and non- parametric tests as appropriate and categorical variables using Chi-squared.
- Kaplan-Meier estimates of survival will be generated based on achieved LDL-C levels and the genotypes, where known.
- Key subgroups of interest will be geographical region, gender, ethnicity, age at baseline, age at risk (if possible), age at first treatment initiation, genotype, comorbid medical conditions (diabetes, hypertension, family history of CVD), and main treatment provider (i.e. whether generalist or specialist).
- To assess whether genotype offers any additional information to risk stratification beyond LDL-C levels in multivariable models of calibration, discrimination and reclassification.
Registered on ClincialTrials.gov
The EAS FHSC Global Registry is now registered as an observational study (Identifier: NCT04272697) on the US National Library of Medicine’s public-facing website ClinicalTrials.gov, to increase public awareness of the project, ethical compliance (though registration is not mandatory for observational studies) and transparency.
Reasons to participate in the FHSC Registry
- Inclusion in a global network of FH experts who share experience and information
- Support to collaborators to establish or develop an FH registry in their country
- Participation in the EAS annual congress and the annual FHSC Steering Committee Meeting
- Information and educational materials on FH
- Support to awareness-raising activities at national level among health care professionals and the wider scientific community
- Free user account for FHSC IDEAPP to support local data entry, management and sharing with the FHSC Global Registry.